Background: Interferon gamma (IFNγ) is a critical cytokine known for its diverse roles in immune regulation, inflammation, and tumor surveillance. Studies have reported the dichotomous nature of IFNγ signaling in both the pathogenesis of cancer and immunotherapy response. However, its complex interplay with acute myeloid leukemia (AML) remains insufficiently understood.
Methods: Three independent RNA-seq datasets were integrated to disentangle the IFNγ signaling in AML. We also performed single cell RNA sequencing (scRNA) on 20 newly diagnosed AML patients to discern relative contribution of cells and cellular communications for IFNγ signaling.
Results: Single-sample gene set enrichment analysis was used to examine the transcriptional programs linked to IFNγ signaling in 672 newly diagnosed adult AML patients. We observed higher IFNγ signaling score in subgroups of patients including diploid with monocytic differentiation, inv16 core-binding-factor AML, and del7/7q. Notably, sorted CD34 + cells from 17 healthy donors had markedly lower levels of IFNγ signaling scores than did those from AML patients, making it a predominant feature in AML. Additionally, we found significant positive correlations between IFNγ signaling score and expression of its downstream targets HLA class 1 and 2 as well as T cell dysfunction score, T cell exhaustion score, and T cell senescence score, consistent with the fact that chronic IFNγ can drive T cell dysfunction.
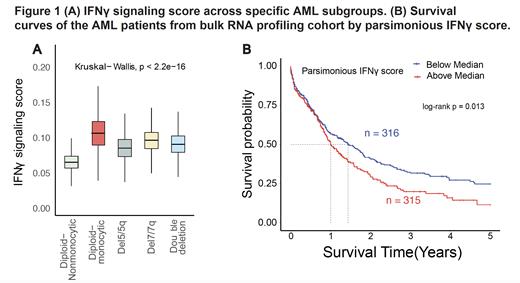
In scRNA, we observed that AML cells in patients with diploid monocytic AML had the highest expression of IFNγ signaling followed by del7/7q, while the non-monocytic, diploid AML cells had the lowest (Figure 1A). We then explored whether IFNγ signaling is differentially activated across the AML hierarchies and found the spectrum along the hierarchy with the primitive cells displaying significant higher IFNγ signaling than GMP while reaching the highest in Mono-like state. Transcription factor analysis revealed high regulon activity of interferon regulator factors (IRFs) in AML cells, with elevated levels of IRF1 and IRF5 regulons in del7/7q and del5/5q, respectively and elevated IRF8 regulon in diploid AML cells with monocytic differentiation, consistent with its role as a lineage-determinant factor promoting monocytic differentiation. Cell-cell interactions revealed IFNγ from CD8 T cells and NK cells as a top interaction to AML cells. These observations indicate that the activation levels of IFNγ signaling in AML cells are associated with distinct cellular states and hierarchies, and that disparate regulons of IFNγ signaling characterize distinct patient subgroups.
The high level of IFNγ signaling in monocytic patient prompted us to assess its correlation with drug response as monocytic subclones are suggested to have inherent resistance to venetoclax. In BEAT-AML ex vivo drug test data, we found a strong positive correlation between IFNγ signaling score and venetoclax resistance, indicating that IFNγ signaling confers venetoclax resistance. This correlation was validated in an independent cohort. However, the IFNγ signaling score did not predict survival outcomes in our bulk cohort. Therefore, we defined a light weighted IFNγ signature to improve the prognostic sensitivity using the least absolute shrinkage and selection operator model. After regression, 47 genes related to survival were retained, forming a parsimonious IFNγ signature. The new parsimonious IFNγ score revealed a tight positive correlation with HLA class 1 and 2 scores. Importantly, this parsimonious IFNγ score was able to predict patient outcomes in our bulk cohort, whereby a higher score predicted worse survival (Figure 1B). Ultimately, these results suggest that the IFNγ pathway activation is associated with resistance to venetoclax-based therapy and can predict patient outcomes independently of known risk factors, making it a promising target for therapeutic intervention.
Conclusions: Characterization of inflammation in AML using independent bulk and scRNA profiling led to the identification of novel drug targets and mechanisms of resistance to targeted therapy. We identified monocytic AML as having a unique microenvironment characterized by high IFNγsignaling in AML cells and immunosuppressive features. IFNγ signaling scores correlated strongly with venetoclax resistance. A parsimonious IFNγ gene signature demonstrated robust prognostic value.
Disclosures
Veletic:Avilect Biosciences: Research Funding. Daver:Hanmi: Research Funding; FATE: Research Funding; Trovagene: Research Funding; Novartis: Consultancy; Trillium: Consultancy, Research Funding; AROG: Consultancy; Syndax: Consultancy; Amgen: Consultancy, Research Funding; Servier: Consultancy, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Jazz: Consultancy; Celgene: Consultancy; ImmunoGen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Glycomimetics: Research Funding; Agios: Consultancy; Shattuck Labs: Consultancy; Kite, a Gilead company: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Kronos Bio: Research Funding. Green:Abbvie: Honoraria; BMS: Consultancy; KDAc Therapeutics: Current equity holder in private company; Sanofi: Research Funding; Abbvie: Research Funding; Kite/Gilead: Research Funding; Allogene: Research Funding; Daiichi Sankyo: Honoraria. Konopleva:Abbvie, Allogene Therapeutics, Cellectis, Forty Seven, Gilead Sciences, Genentech, Sanofi, MEI Pharma, Rafael Pharmaceuticals, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini, Precision BioSciences.: Research Funding; AbbVie, Forty Seven, Precision Biosciences, Gilead Sciences, Genentech, Janssen, Sanofi, MEI Pharma, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini.: Consultancy; Reata Pharmaceuticals.: Current holder of stock options in a privately-held company, Patents & Royalties. Post:LinBioscience: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal